Developement and Validation of Single Residue Methods - Strategy of EURL-SRM
Main Challenges
The development of methods for pesticides traditionally requiring single residue methods is one of the most time-consuming and challenging activities of the EURL-SRM.
One of the main challenges to face in this respect is that most laboratories are generally very reluctant in introducing SRM-pesticides in their scopes. As a result very limited data exist as regards the residue situation of many SRM-pesticides in food products from the market.
Traditional single residue methods (SRMs) are typically as work- and cost-intensive as multiresidue methods (MRMs), but cover only one or a limited number of pesticides. Introducing an SRM-pesticide in the scope of a laboratory typically requires much more effort than including an MRM-compound in an existing method.
The decision to introduce and run an SRM-method in a lab does thus not only depend on the availability of the necessary instrumentation but also on whether the the effort-to-benefit ratio resulting from this step is favourable enough for the lab. The effort within this context results from the additional work involved in implementing and validating the new method and in subsequently applying it on a routine basis, which essentially boils down to additional ressources of personnel and instrumentation being necessary. The benefit results from producing residue data for one or more pesticides that pose a certain risk to the consumers.
5-Steps-Strategy
Having all these considerations in mind, the EURL-SRM pursues the following 5-steps-strategy to increase the benefit, reduce the effort and give the laboratories additional incentives to implement selected SRM-pesticides in their scope:
- Risk-based prioritization of the SRM-pesticides using the pesticide ranking list developed by the EURL-SRM
- Development of simple and attractive methods for high priority SRM-pesticides. In order to be easily transferable to other labs, commonly available instrumentation (mainly LC-MS/MS under standard configuration) is to be preferred. Isotopically labelled standards are applied wherever possible to further simplify the procedures. Where no such standards are available, manufacturers are contacted to synthesize them and make them available to the labs. To increase the effort-to-benefit ratio and attractiveness of the methods as many as possible pesticides with similar properties are implemented in one single residue method.
Where pesticides can be analyzed by modifying an exiting MRM (QuEChERS) such modified MRMs are preferably developed (e.g. acidic pesticides, PCP, Nicotine) - Distribution of method protocols via the website (open-link) and further promotion via oral presentations and training courses during the EURL/NRL training-workshops as well as in conferences.
- Gradual inclusion of selected high-priority pesticides in the multi-annual CCP plans;
- Gradual inclusion of selected high-priority pesticides in the EUPT-SRMs.
It should be noted, however, that even if these criteria are met, the implementation of a SRM-method in a given lab will always depend on the situation within the lab with factors such as available personnel and instrumental capacity as well as motivation and energy of the lab-staff playing a decisive role. According to Doc. No. SANCO/10684/2009 labs are free to use any method they like as long as the stipulated performance criteria are met. The methods provided by the EURLs are to be considered as a service to OLs rather than as a prescription on how to perform analyses.
All methods developed by the the EURL-SRM have been validated internally. The method for acidic pesticides has been validated in an inter-laboratory study. The same is aimed for the method for polar pesticides with the intention to establish it as a CEN-procedure.
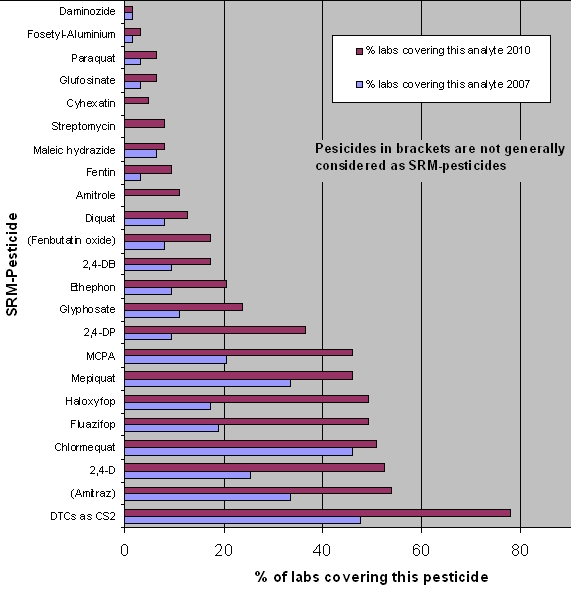
The EURL-SRM has distributed methods for all pesticides shown in this graph:
Published 24-08-2010, 13:45:12
Top of Page

